RPL – Research Program Lines
Vesicular precision medicine: A trojan horse at the nanoscale
People worldwide are living longer. With the increasing life expectancy of the world’s population, also the frequency of chronic diseases such as cancer, neurodegenerative, and chronic inflammatory conditions, as well as functional defects of the musculoskeletal system, is raised since their risk increases with extended life expectancy. The WHO has recognized this serious public health issue as it limits the quality of life of the individual and poses a significant financial burden on healthcare systems. Managing and treating late adult-onset disorders is currently limited by the lack of safe and effective therapeutic options that are precisely targeted to the patient’s situation. Future breakthroughs are expected from approaches that take advantage of natural systems and turn them into drugs, such as therapeutic antibodies, nucleic acids, gene editing, or cell therapy. However, many of these new modalities show poor bioavailability, severely limiting their clinical application.
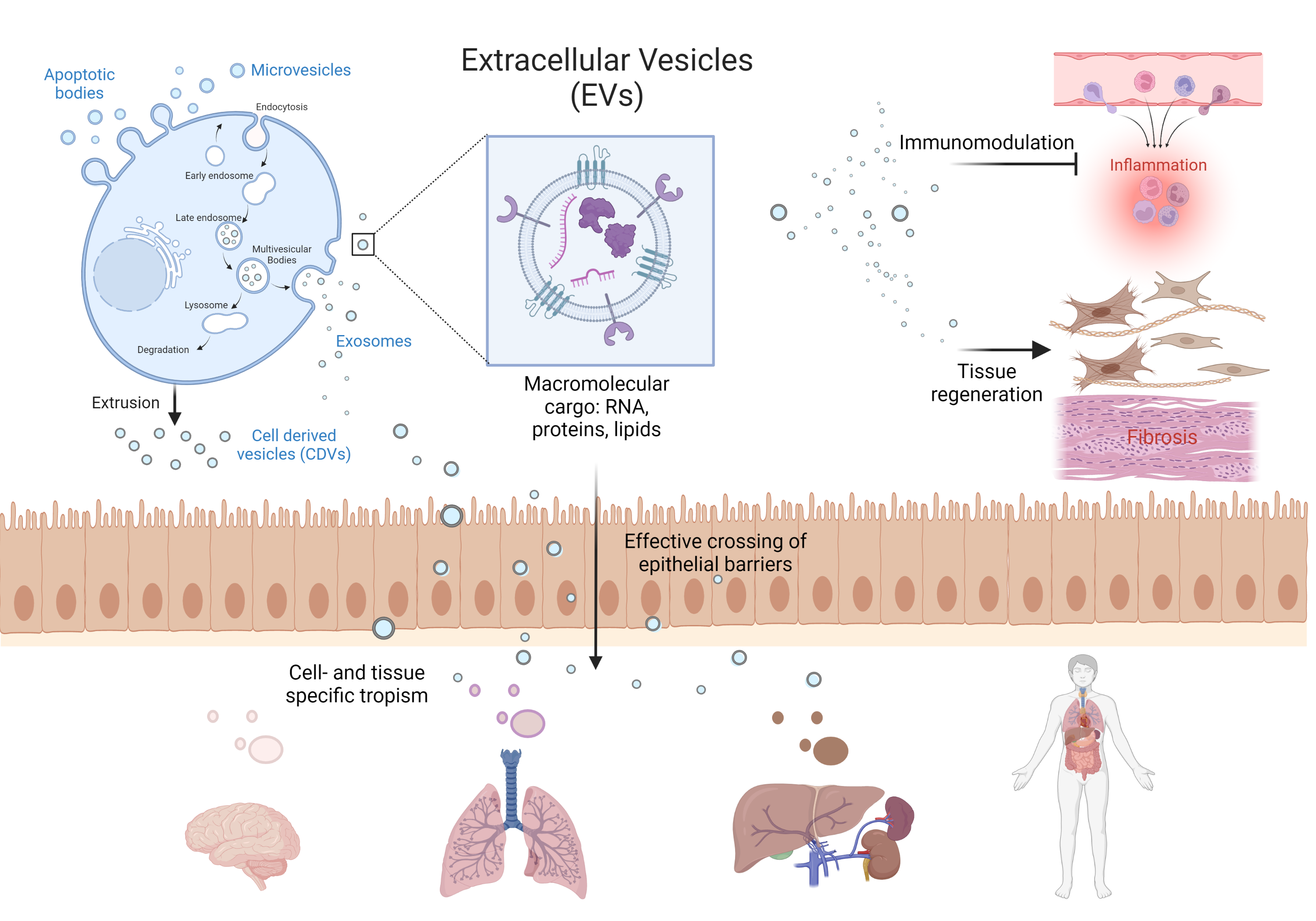
Nature´s own solution to this very problem is secreted nanovesicles (NVs) that continuously circulate the body to transfer molecular cargo between cells, tissues, and organisms with high efficiency and precision, affecting the fate and function of their recipient cells (Figure 1). Utilizing this evolutionarily established trafficking system may enable the effective delivery of non-drug like molecules across biological barriers, specifically targeting diseased cells and tissues while being highly biocompatible. Despite the disruptive biomedical potential, several essential scientific, technological, and practical challenges remain to be resolved.
The Ludwig Boltzmann Institute for Nanovesicular Precision Medicine (LBI NVPM) has a mission to turn the small new world of nanovesicles into precision medicines. LBI NVPM will develop a next generation drug delivery platform to enable safe, effective, and targeted delivery of new therapeutic modalities (RNA, gene editing RNPs, heterobifunctional ligands). The innate tropism of NVs from different sources will be tailored to specifically target the affected tissues. We will develop novel strategies for cargo loading into NVs, and processes for economically and environmentally sustainable scale manufacturing from industrial waste streams. The primary goal are therapeutic candidates for cancer and neurodegenerative diseases, with a focus on oral administration using food derived NVs.
The inherent anti-inflammatory and pro-regenerative activities of NVs from human primary stem cells, platelets, and milk will further be translated into novel treatments for local and systemic immune cell modulation and tissue repair. Focus areas or our research include rheumatoid arthritis, implant-induced foreign body reactions, tendon diseases, and chronic postoperative neuropathic pain suitable for topical application and exploiting novel clinical scaffolds.
Our two biomedical research programs are supported by application-guided nanovesicular technology development, delivering (i) a comprehensive catalogue of quantitative bioanalytics for in-depth NV characterization and quality control, (ii) advanced imaging technologies, and (iii) label-free approaches to quantitatively track the spatio-temporal distribution of NVs and their cargo in vivo. We will develop minimally invasive biomarkers for clinical monitoring, companion diagnostics, and the prediction of therapeutic responses to NV drugs.
Effective clinical translation of the developed NV therapeutics will capitalise on our unique GMP manufacturing environment and expertise, and we aim to maximise the impact by active integration of patients, healthcare professionals, diagnostic centers, industry, regulators, policymakers, and the broader community. Ultimately, the mission of LBI NVPM is to provide direct benefits to society by (1) delivering safe, effective, and well-tolerated NV precision medicines, improving the quality of life of patients suffering from chronic and adult-onset diseases, (2) alleviating the burden on the healthcare systems, while (3) pharmaceutical use of side streams from industrial food processing will contribute to sustainable use of resources.